SO, today we learned about three types of reactions: Synthesis, Decompositions, and Single Replacement.
Synthesis

What you see above is an example of a synthesis reaction. A synthesis reaction is a reaction that combines two or more reactants to form one product.
The general formula is:
A + B = AB
Lets use an example that doesn't involve starving animals:
Ex 1/ 8 Fe + 1 S8 -> 8 FeS
Ex2/ 2 Na + 1 Cl2 -> 2 NaCl
Decomposition
Egg -> Shell + Turtle
A decomposition reaction is a reaction that breaks down one reactant into two or more products.
The general formula is:
A -> B + C
Ex 1/ 2 NaCl -> 2 Na + 1 Cl2
Ex 2/ 1 MgS -> 1 Mg + 1 S
Single Replacement
Hot guy + Scrawny Guy and Girl = Hot guy and Girl + Scrawny Guy
A single replacement reaction is one where an element replaces an ion in an ionic compound. Metal elements replace positive ions and non-metal elements replace negative ions.
The general formula is:
A + BC -> AC + B (A = metal)
A + BC -> BA + C (A = non-metal)
Ex 1/ 3 Ba + 1 Ni3(PO4)2 -> 3 Ni + 1 Ba3(PO4)2
Ex 2/ 2 Hg + 1 Sn(SO4)2 -> 1 Sn + 2 HgSO4
After we learned this, our lovely teacher gave us a WONDERFUL sheet called the "Activity Series". This table helps to predict whether the single replacement has a reaction or not. Before, we just assumed that it did but NOW, our whole perspective on life has changed.
Some metals are more reactive than other metals, and similarly some non-metals are more reactive than other non-metals
So, to read the "Activity Series", understand that an element higher up on the series replaces the ion below it on the table.
If the reactant is higher than the product, then it creates a reaction.
Ex / 2 Fe + 3 CuCl2 -> 3 Cu + 2 FeCl3
Look at Cu and Fe. Which one is higher on the table? Iron is higher, and iron is the product, meaning there IS a reaction.
Ex/ 1 Cl2 + 2 KBr -> 2 Br2 + 1 KCl
Look at Br and Cl. Which one is higher on the table? Chlorine is higher, and chlorine is the reactant, meaning there IS NOT a reaction. Too bad so sad.
This is a tutorial for single replacement reactions
Well thats all for now people : )
Peace.
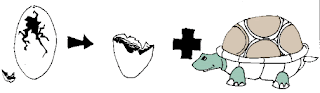

No comments:
Post a Comment