WE ARE THE SMARTICLE PARTICLES. We are composed of three pretty awesome people named Melissa Jessica and Hanae.
Wednesday, October 27, 2010
Seperating All The Wonderful Glories of Chemistry
So today we will be learning about the joys of Separation Techniques. There are five main types we will be learning about today:
-Filtration
-Floatation
-Crystallization and Extraction
-Distillation
-Chromatography.
Scratch that, there are six, I think.
Let's first talk about Hand Separation and Evaporation. A few key points to remember:
-This is when you try and separate solids from solids.
-Magnetic things can be separated using a magnet.
Ex; There are a bunch of coins and you want to separate the pennies from everything else.
- You'd obviously just use your hands and pick the pennies out.
-Evaporation, I'm sure you all know is when you heat up liquids so you get the solid residue/remains.
-Evaporation is used when there is a solid dissolved in liquid solution.
Ex; When you have salt water and you only want the salt.
Second method, (gosh I'm tired already) Filtration
-You can use filtration when there is an undissolved solid inside a liquid.
-You'd use a porous filter to separate the two matters.
-If the pores of the filter are smaller than the particles themselves, obviously the solid stuff will stay on the filter while all the gas and liquid passes through it; solid particles are too rigid to pass through the pores.
-You'd usually use filter paper to do all this.
Third method (trust me, this kills me as much as it kills you), Crystallization
-Use this method when you are separating a solid from a liquid.
-First you'd have to convert the solid into a solute. The solute is usually made via a chemical or physical change.
-After converting the solid into a solute, you'd then separate it using the filtration or floatation method.
-Finally, you evaporate or cool down the substance until the solid comes out as pure crystals. If needed, you can filter the crystals from the remaining solvent.
Fourth Method (I should really get to PHYSICS soon...), Gravity Separation
-This is a pretty straightforward method.
-When you use gravity to separate things based on their density.
-Usually used to separate solids from solids.
-Something called a centrifuge helps do this, and whirls a test tube around at a super high speed making the denser substance sink to the bottom.
-Works most effectively when done with small amounts of material.
Fifth Method! (back to enthusiasm! Yay chem!), Solvent Extraction.
-This is when a component wafts into a solvent when it is shaken with the mixture.
-This works effectively when done with solvents that only dissolve one component.
-When it is a MechanicalMixture, a solid mixed with a solid, you use liquid to dissolve ONE of the solids. The solid is then left behind or dissolved.
-But oh, what if the solvent is insoluble?
-The liquid will then form layers and drain the insoluble solvent.
-This leaves the unwanted substance (solid) behind.
Sixth Method (Not going to lie,I just got distracted by Facebook), Distillation.
-Have you ever seen "distilled water" on your water bottle? Well I'm going to educate you about that.
-You heat the mixture, and substances with a low boiling point vapourizes first.
-Essentially, it is the collecting and condensing of vaporized components.
<---------Diagram.
Seventh and final Method!!...Split into two parts. Chromatography.
-Two types of chromatography:
-Paper Chromatography (PC)
-Thin Layer Chromatography (TLC)
-A mobile phase wooshes the sample over a stationary phase. (graphically speaking)
-Separates complex mixtures like drugs, plastics.
-A highly accurate and precise method.
-Once separated, you can collect the components individually. So basically, it's convenient.
Sheet Chromatography.
-PAPER CHROMATOGRAPHY:
-Stationary Phase is a liquid drenched into a sheet of paper.
-Mobile Phase is simply a liquid solvent.
-Some components take longer in the stationary phase.
-Appears in separate spots on the paper after separation is complete. Or after "developing"
-THIN LAYER CHROMATOGRAPHY:
-Stationary Phase; a very thin layer of absorbent. It usually coats some sort of sheet.
-Ditto Paper Chromatography, the "developed products" appear on various spots on the sheet after procedure is complete.
Monday, October 25, 2010
Read on for the Scariest and most FREAKY story you have ever heard. It is called....THE NAMING OF ACIDS.
Well my friends..............I am having that exact problem right now. But, I will try. Feel free to add in any shrieks and hollers for EMPHASIS...a.k.a making me feel better. ALL RIGHT let's get started
*puts flashlight to face*
It was a dark grey spookey night. _______ (insert your name here) was wandering through the woods ALONE at night on halloween. (You aren't that smart I guess)

Every step you take leads you closer to the dangers that lie ahead. You ignore the noises you hear from the bushes beside you..thinking it is just the wind, BUT soon enough you realize, THERE IS NO WIND.
You whip around looking everywhere to see who is the source of the noise, then suddenly, a man POPS OUT OF THE BUSHES.
He has the word "Acid" printed across his shirt.
"Who are you?" you say trembling.
"Why, I am ACID MAN!" the guys screams. "I CORRODE AND BURN YOUR SKIN AND TASTE SOUR"
"NOOOOO" you scream. "Is there any way I can convince you not to burn me?"
"Well, there is ONE way, but NO ONE has completed this task before. All you have to do, is explain to me the different ways of naming SIMPLE and COMPLEX acids. Then I will let you free. You see, no one knows how to name me nowadays." he says.
"I will take that challenge" You reply, remembering that you have learned this lesson already in chemistry class.
So, those who do not know how to name acids will get burned. For those of you that do, GOOD JOB, you are free to go : D
Okay fine, I'll help you guys out, after all, we need people to continue reading our blog, we can't just let acid man burn all of you. pshht.
An acid is a covalent bond that is formed by a hydrogen ion and a negatively charged ion dissolved in water. The ions separate when they are dissolved in water.
Chemical formulas for acids, start with the letter H which stands for? No its not habanera, harmonometer, or hygeiolatry (if you guys even know what those mean..I do..hehe). Well the letter H stands for Hydrogen in the formula.

First, let's start by naming simple acids.
These always start using the prefix: hydro
But after hydro, you need the name of the negatively charged ion. You can't have something like hydrobiological and say its an acid.
Examples of simple acids are:
HBr
HS
HF
Now, to name these, the negatively charged ions need to end using "ic" then write acid after this. Soo...
HBr = Hydrobromic acid
HS = Hydrosulphuric acid
HF = Hydrofluoric acid
Simple enough?
Now we're moving on to the complex acids. This is where it starts getting tricky.
If the polyatomic anion ends in "ate" you need to change the end to "ic"
If the polyatomic anion ends in "ite" you need to change the end to "ous"
An EASY was to remember this is to say in your head:
I "ate" "ic" and got "ite" "ous"
orr what we learned in ms chen's class
I "ate" "ic"-y sushi and got appendic "ite" "ous"
Oh wait, they're basically the same...but one's longer. Oh well.
Some examples of complex acids are:
HNO3
HNO2
HCH3COO
Now to name these, instead of starting with hydro, you need to start with the name of the polyatomic anion with the new ending then write acid after it. SO:
HNO3 = Nitric Acid
HNO2 = Nitrous Acid
HCH3COO = Acetic Acid
The only exception to these rules is the acid HCN. This is a simple acid so it is named hydrocyanic acid.
NOW, TELL THAT TO THE ACID MAN.
Well, my fingers are tired now, so basically you know what happens next in the story. You tell acid man and he lets you free then you live happily ever after yada yada yada.
THE END.
Wednesday, October 20, 2010
A Fun, Fantastic, and Fulfilling Day with PAPER CHROMATOGRAPHY!
Ooooh. Sorry. Did I cross the line? Okay, I'll stop. :S
THE GENUINE AND SOPHISTICATED LAB SUMMARY BEGINS NOW.
On Tuesday, October 19th, 2010. We performed Lab 3B out of Essential Experiments for Chemistry. This is page 32-37 of the book.
The idea of the lab was to discover the components and Front Ratio (Rf) of various solutes (food coloring) with a solvent (water). We were to do this through paper chromatography, of course.
I hope that you all remember what was done during the lab. For example, the observations you made, the type of data collected, and the calculations made.
Recall that we drew a pencil line across each of our three 22cm strips of chromatography paper, 4 cm from the end, and cut it so that the strip has a pointed tip.
After putting a drop of sample onto the drawn line of the strip, we placed it into a test tube with 2cm of water. After 20 minutes of waiting, we measured the distance between the length travelled by the solute (d1) by the solvent (d2) from the pencil line. (Measurements were done using a ruler, measuring in cm) We then calculated the component:solvent front ratio (Rf).
The way to calculate the front ratio was simply to divide the distance that the solute travelled by the distance that the solvent traveled. Your result (aka. your Front Ratio, Rf) should be between 0 to 1. This "rule" can be found in your lab book!
Remember all the observations you saw while the fronts moved. Certain colors of the solute seperated into multiple colors, such as blue, yellow, and red! However, some solutes, such as the one with yellow coloring- did not seperate! It just remained the same color except expanded. This is because there are no colors that mix together to create yellow. But, yellow can be found as a component, because it is used in mixing to create new colors.
This lab should've helped you understand a very accurate form of seperation, which again, is called paper chromatography!

Monday, October 18, 2010
Review from Science 10 when we were Oh So young and uncivilized: Writing + Naming Ionic and Covalent Compounds
The lesson today was on writing and naming Ionic and Covalent Compounds (as you can probably plainly see from the title). Does it sound fascinating? Well GOLLY GEE it sure was.
Well, lets start off with explaining what ions are. Ions are atoms or groups of atoms that are positively or negatively charged. Basically, they are composed of two or more oppositely charged particles (which are ions). They are held together by electrostatic forces (ooo :D)
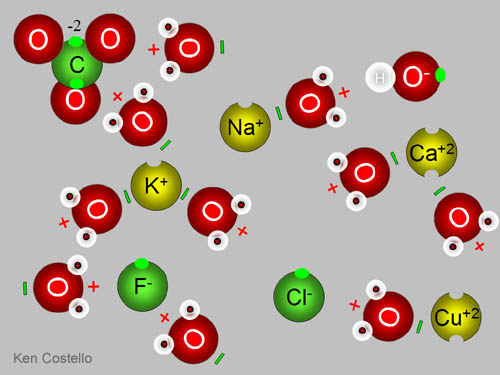
In ionic compounds, electrons are transferred from a metal to a non metal. When writing Ionic Compounds, I LOVE doing the switch method. It's the easiest to understand. Hehe. What happens is the two particles switch charges.
For Example:
Strontium Fluoride
Strontium has a charge of +2
Fluoride has a charge of -1
So, that's Sr2F. BUT since they switch charges, it becomes SrF2
Simple enough? Well too bad, now it gets harder. If the charges are the same, they both canacel out.
For example:
Calcium Sulphide
Calcium has a charge of +2
Sulphur has a charge of -2
When this happens, DO NOT (under any circumstances, even if your dog is dying) write Ca2S2. WRONG WRONG WRONG WRONG WRONG.
The two's cancel out and that makes CaS.
Lastly, you need to know what happens when you have numbers that can easily divide into eachother
For Example:
Rhodium (IV) Oxide
Rhodium has a charge of +4
Oxygen has a charge of -2
So the equation is Rh2O4. BUT *gasp* there is a catch, this formula can reduce!!!
2 can easily divide into 4 because 4/2 = 2 (I hope you already know that but if you're suddenly brain dead, I wrote it out for you. It's okay, I have brain farts all the time).
SOOO...the formula becomes RhO2 because the simplifying cancelled out the 2 on rhodium and made the 4 a 2 on oxygen.
You see how that works? MAGICAL!
So now, we can finally move on to naming Ionic Compounds =.=
Okay so just to review a bit from last year, the metal is ALWAYS first and the non-metal is ALWAYS second. (Don't you feel bad that it always has to come in second? D:)
When naming, you need to change the ending of the non-metals to "ide"
For example:
Sulphur becomes Sulphide
Oxygen becomes Oxide
Fluorine becomes Fluoride
And it goes on and on and on and ooonn, strangers, waiting, up and down the boulevaarrdd. (okay i got carried away).
When the particle has more than 1 charge, you need to state the charge in roman numerals after the name of the particle.
For example:
FeO = Iron (II) Oxide
PbCl4 = Lead (IV) Chloride
If you do not remember your roman numerals (which I totally understand) I will be kind enough to write them out for you :)
1 = I 3 = III 5 = V
2 = II 4 = IV 6 = VI
Okay guys, we're now moving on to covalent compounds oooo, ahhh, ohhh. yes.
So covalent compounds, unlike ionic compounds, are between a non metal and non metal. They share electrons. Writing covalent compounds have the same rules as writing ionic compounds, but naming them is a different story.
Covalent compounds use Greek prefixes to indicate the number of atoms. Ohoho what is this? It appears we are taking a trip back YET AGAIN to ancient greece.
To truly understand the naming of covalent compounds, we must understand the Greek ways of numbering.
Mono = 1 Penta = 5 Nona = 9
Di = 2 Hexa = 6 Deca = 10
Tri = 3 Hepta = 7
Tetra = 4 Octa = 8
You need to put the greek prefixes infront of the particles names.
For example:
NH3 = Nitrogen trihydride
You do not need to put a "mono" infront of nitrogen even though it only has one atom, because you don't need to for the first particle.
The last thing you folks need to remember is the Diatomic Molecules. These are:
Hydrogen, Oxygen, Fluorine, Bromine, Iodine, Nitrogen, and Chlorine.
OR better known as
HOFBrINCl
(prounounced Hoffbrinkle)
These all have two atoms:
H2, O2, F2, Br2, I2, N2, Cl2
When named, you must put a "gas" behind them.
For Example:
H2 = Hydrogen Gas
O2 = Oxygen Gas
F2 = Fluorine Gas
WELL THANKS FOR READING. I KNOW IT WAS A LOT. Now you may rest your eyes from staring at this screen. Researchers say it is bad to stare at a computer for more than two hours at a time. o.o
WELL BYE.
peace.
Monday, October 4, 2010